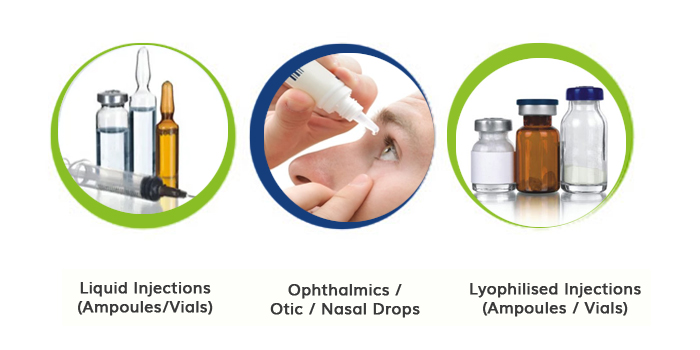
Introducing Cirons' cutting-edge sterile manufacturing facility specializing in Small Volume Injectable & Ophthalmic products, meticulously designed to meet the stringent standards of USFDA, UK-MHRA, EU, Brazil, Mexico and PIC/S GMP regulations.
Our 3rd manufacturing facility is poised to serve the demands of regulated and PIC/S markets with unrivalled precision and innovation..